We translate science into treatment.
With patients for patients –
rethinking clinical trials.
We are a full-service CRO with an in-house site network
CTC stands out by its in-house Site Network with 6 state-of-the-art research clinics across Sweden, including a FIH unit at Uppsala University Hospital, and an additional clinic in the Netherlands.
We provide full-service management and conduct of Phase 0, FIH, Phase I and Phase II studies as well as medical device investigations. Our site network supports clinical trials in all phases of development. With a flexible and innovative mindset, we offer a streamlined, cost-effective, and optimised solution customised for each trial.
We translate science into treatment.
With patients for patients – rethinking clinical trials.
Company structure
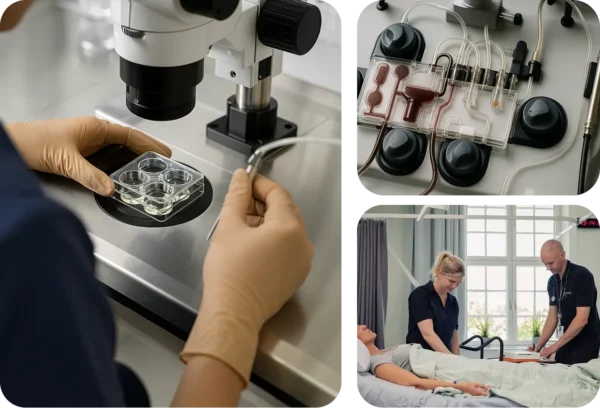

In-house Site Network for Phase 0 to Phase IV studies
Medical and Scientific Advice from pre-clinical through clinical research
beds
in-patient stays
out-patient visits
clinical trials
FIH trials annually
blood samples annually
Service portfolio
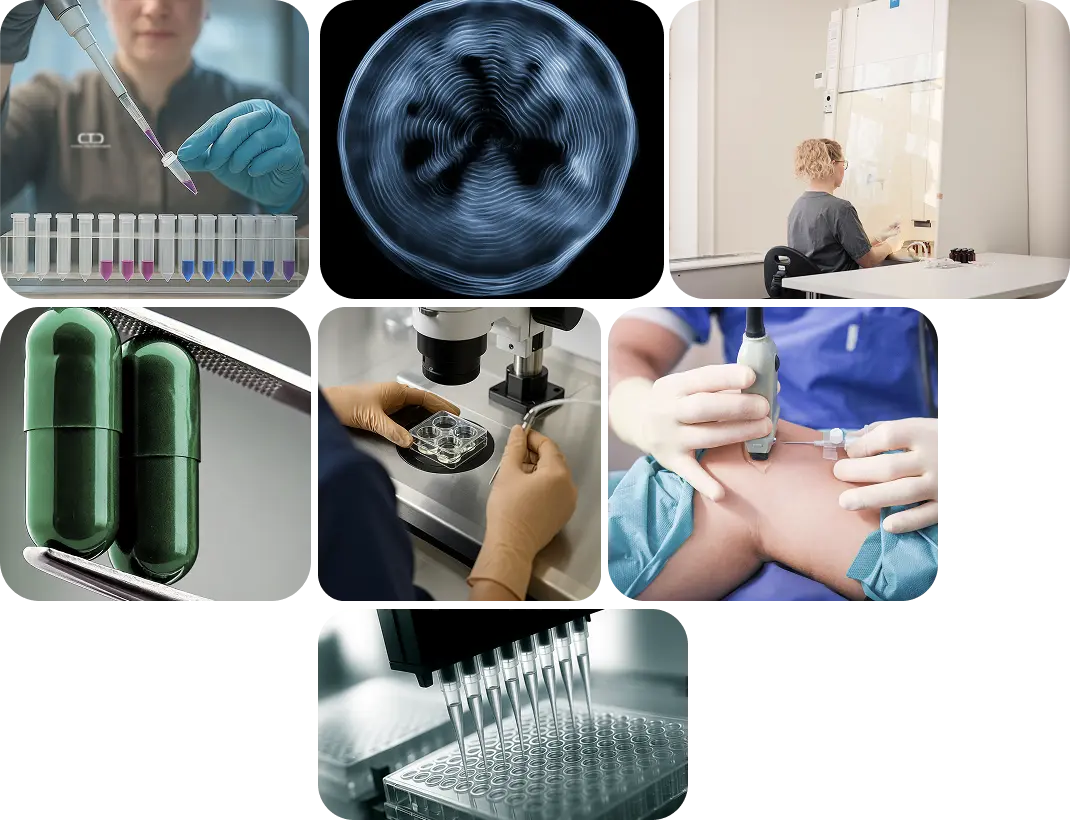
CTC’s comprehensive service portfolio is built upon our in-house expertise in Project Management, Clinical Monitoring, Medical Writing, DMPK & Clinical Pharmacology, Biometrics and pre- and post-market Pharmacovigilance. Additionally, our Site Network offers 6 in-house state-of-the-art research clinics strategically located across Sweden and an additional research clinic in the Netherlands.
Special techniques
CTC is committed to advancing clinical trials through innovative methodologies and employs cutting-edge techniques that not only allows for smart trial design to address complex endpoints but also facilitate the execution of decentralised trials.

More than 140 co-workers constitute the backbone of our company
We believe that our workplace should not only be full of expertise but also of warmth, transparency, and flexibility. A place that is open for fast adaptation to new, innovative techniques. Committed to our work, we aim for high-quality deliverables that will meet our clients’ expectations.
We provide our customers with cost-effective advice, conduct and reporting of clinical trials.
Testimonials
"CTC provided us with excellent service during our phase 1 study in volunteers. CTC is flexible, service-minded and experienced."

Jimmy Gu
ArkBio
"The entire study has been conducted to a very high standard, and I’m very pleased with how smoothly everything has gone. Thank you for always taking onboard our feedback and suggestions, and for being so collaborative. I know that you take pride in your work – and it is very much appreciated. It has been a pleasure to work with you. I’m really hoping that we can place another study with CTC."
Dr. Kate Hanrott
Director of Clinical Operations and Sciences, EpiEndo
"Asarina Pharma has used the PV and medical monitoring services from CTC. Everything has gone very smoothly, professionally and flexible; all the way from setting up the safety management plan to safety reporting and follow-ups. We would do it again!"

Märta Segerdahl
CMO, Asarina Pharma
"CTC’s recruitment team has done an absolutely fantastic job! In the middle of the corona pandemic’s most uncertain initial phase, we started a clinical study and enrollment is completed by a full month’s margin."

Arvid Söderhäll
CEO, Empros Pharma


